(Upload on February 10 2026) [ 日本語 | English ]
Mount Usu / Sarobetsu post-mined peatland
From left: Crater basin in 1986 and 2006. Cottongrass / Daylily
HOME > Lecture catalog / Research summary > Glossary > Embryology
Developmental biologythe study of the process by which animals and plants grow and develop, including regeneration, asexual reproduction, metamorphosis and the growth and differentiation of stem cellsGrowth and developmentGrowth (成長)irreversible change in mass |
Developmentirreversible change in state
embryogenesis (胚形成) |
|
1677 Leeuwenhoek: discovered sperm by a microscope
Ham, Johan: brought in gonorrhea ejaculate to Leeuwenhoek Preformation and epiformation (前成説と後成説) ☛ view of lifePhylogenetic vs ontogenetic developmentPhylogenetic development - evolution |
Homeobox (ホメオボックス)DNA sequence, ≈ 180 base pairs regulating development and differentiation in the early stages of embryonic developmentGehring, Walter Jakob (1939-2014), Drosophila genetics and development cell determination in the embryo 1983 Gehring et al.: discovered homeobox |
Testis and sperm (精巣・精子)Sperm or spermatozoon (pl. –a) (精子)The morphology is different at species level. Sperms, mostly consisting of nucleus, contain least nutrients but have high mobility.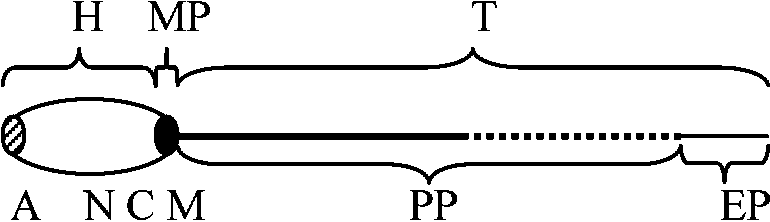 Fig. 32. Diagram of primitive metazoan spermatozoon (Franzen 1956). A: acrosome, H: head piece, M: mitochondrial body, PP: principal piece, MP: middle piece, T: tail section, EP: end piece, C: centriole, N: nucleus.
[Sperms of Gymnosperms: Ginkgo biloba and Cycas revoluta]
Fig. 32. Diagram of primitive metazoan spermatozoon (Franzen 1956). A: acrosome, H: head piece, M: mitochondrial body, PP: principal piece, MP: middle piece, T: tail section, EP: end piece, C: centriole, N: nucleus.
[Sperms of Gymnosperms: Ginkgo biloba and Cycas revoluta]
Egg (卵)Organization of egg cytoplams (卵細胞質)
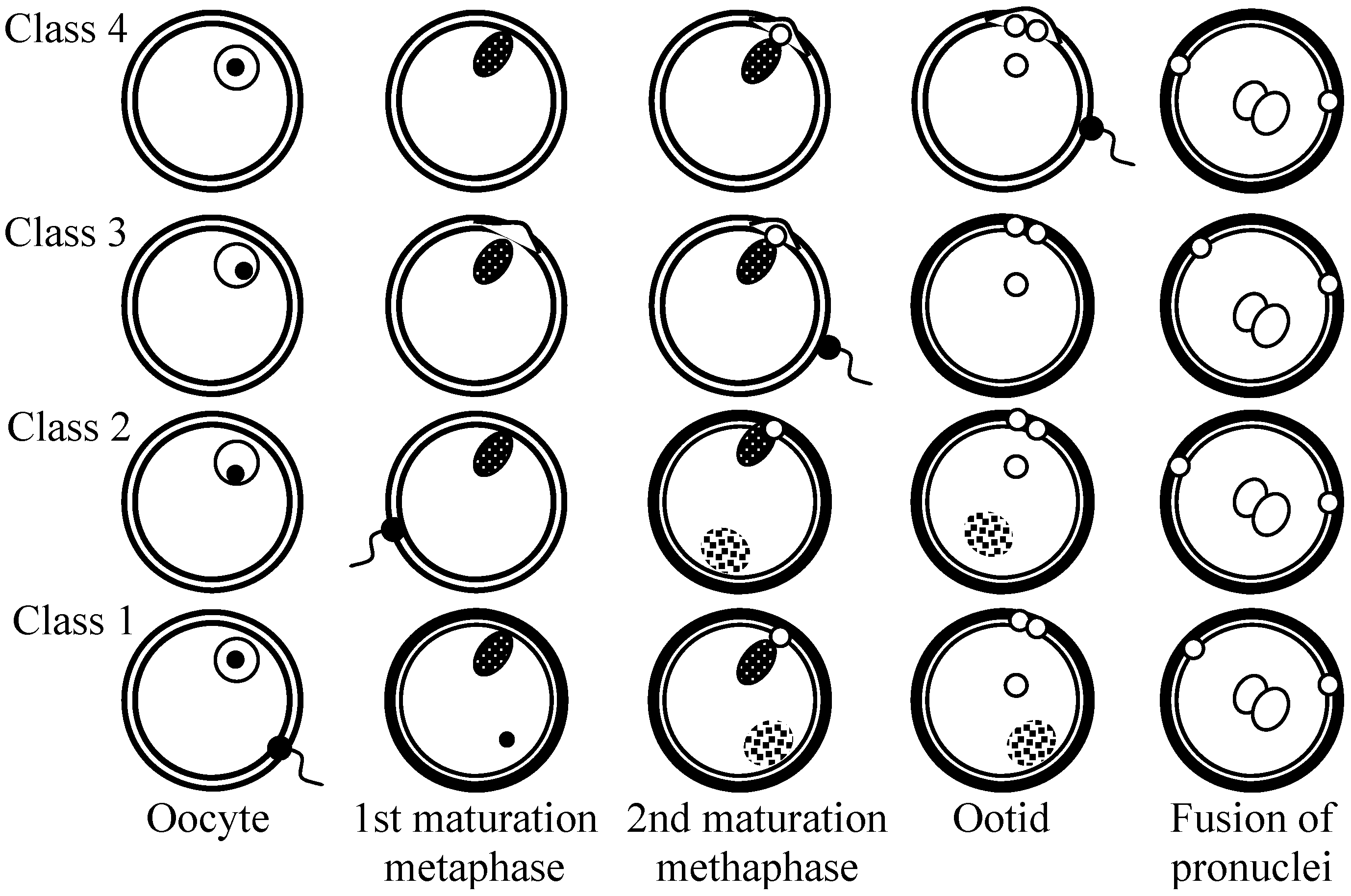 Fig. 1. The four stages of egg maturation at which fertilization occurs in the animal kingdom. (Dalcq 1952) |
Table. Chemical composition of the jelly coat (卵ゼリー) of echinoderm and amphibian eggs (% dry weight). +, qualitative estimation only; ?, to be controlled; G, glucose; Gl, galactose; M, mannose; F, fucose; X, xylose; Fr, fructose; Ga, glucosamine; Gla, galactosamine; TN., total nitrogen.
Species + + + + + 4.6 3.8 24.3 0.8 4.7 20.9 + + 25.0 25 5.7 23 + + + + + + + 32.7 4.1 20.5 8.1 ? 12.7 3.5 7.1 ? 8.9 9.5 8.1 + + + ? 9.3 28.0 ? 14.0 8.8 ? ? ? + + + + + 1.7 16.5 10.0 + + 10.4 20-40 7.6 30.0 ? 20.0 8.4 + + + 8.3 12.2 1.3 6.2 20.3 10.0 Lampbrush chromosome (ランプブラシ染色体)
|
Theories on fertilization
1. Fertilizin theory (Lillie FR) - negative |
(b) → oxidation of unsaaturated fatty acid (Ca-activated enzyme)
→ O2 uptake Table. O2-consumption of unfertilized and fertilized eggsSpecies (Stage of mat. division at the time of fertilization) = (O2 uptake of fertilized egg)/(O2 uptake of unfertilized egg)
Phallusia mamillata = 2.0 N. limbata Germinal vesicle = 1.35 Mactra laterialis = 1.8 Urechis caupo = 1.2 Cumingia tellinoides = 0.45 Chaetopterus variopedatun = 0.53 Marthasteriaa glacialis = 1.0 Saxostrea commercialis (metaphase of 1st mat.) = 1.0 Sabellaria alveolata (division) = 1.1 Asterias glacialis = 1.0 Ciona intestinalis = 1.5 Table. Fertilizability of Asterias eggs at various pH values HCl NaOH
Concentration N/ N/ N/ N/ N/ Normal N/ N/ N/ N/ N/
in sea water 500 1000 2000 4000 104 sea 104 4000 2000 1000 500
water
Cleaved egg % 0 2.5 8 22.5 45 53 84 92.5 88.5 89 0
|
|
Table. Cleavage of frog eggs by the injection of tissue (testis) extract and of various fractions of the same obtained by centrifugation.
Preparation________Eggs reaching blastula stage (%) |
Table. Nucleic acid content in nuclei during gametogenesis and cleavage in Chaetopterus
Amount
Condition of Asymmetric property (非対称性)1928 Spearman
|
Development of gonad (生殖腺発達)1. Origin of primordial germ cell (gamete) (始原生殖細胞の起源)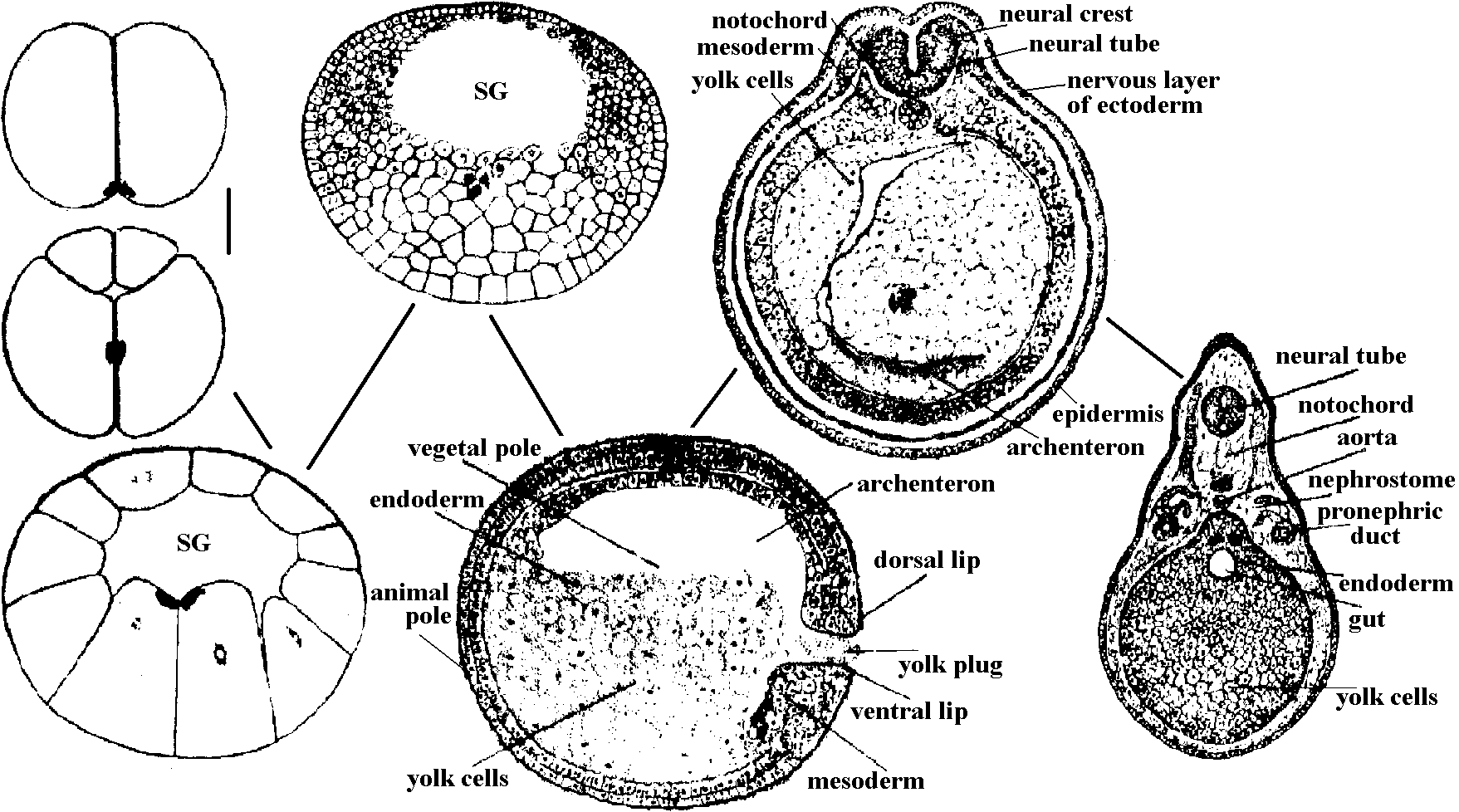 Fig. Diagrams showing the displacement of the germinal cytoplasm during the early development of Bufo regularis. 2. sex differentiation (性分化) |
Pre-embryo (初期胚)= early embryo or nascent embryo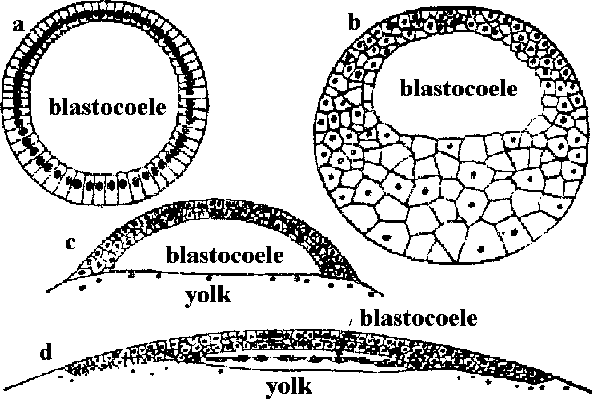 Fig. Diagrammatic comparison of blastulae (胞胚) of an echinoderm (a), a frog (b), a bony fish (c) and a bird (d). |
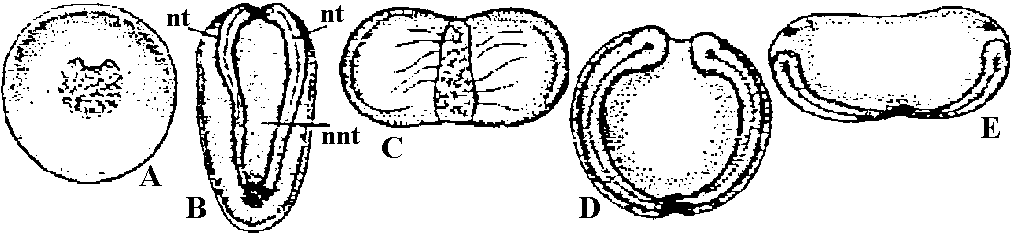 Fig. A, B, development of an embryo with animal pole material graft placed in the grey crescent. A, during gastrulation; B, after neurulation. nt, neural tissue, ntt, non-neural tissue, C, D, E, development of an embryo with grey crescent graft at ventral margin. In C embryo shows two neural plates and dorsal lips, one induced, the other normal, D, E show two extreme types of embryo which are produced by these grafts, after neurulation. 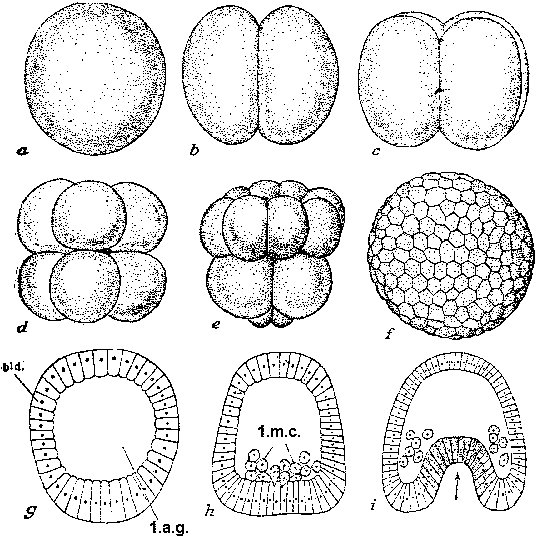 Fig. Segmentation and gastrulation in sea urchin. a) fertilized egg, b) 2 blastomere stage, c) 4 blastomere stage, d) 8 blastomere stage, e) 32 blastomere stage, f) blastula (appearance from above), g) blastula (longitudinal section), h) arising of first mesenchyme cells, i) gastrula occurrence. Bld-blastoderm: 1.a.g. first abdominal gap, 1.m.c. first mesenchyme cells. |
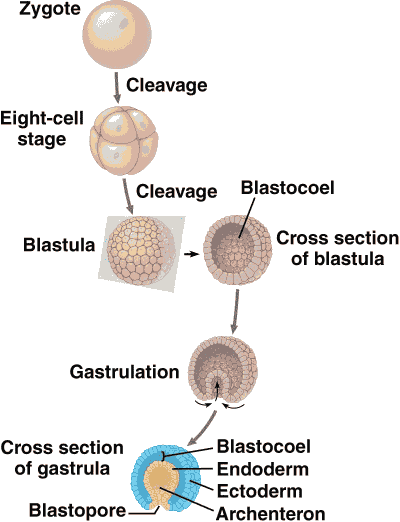 Table. Chemical composition of sea urchin embryo (% wet weight). (subs = substances. carbs = carbohydrates)
substance water dry protein total glycogen fat ash
Substance Egg Hatched tadpole Species Scyllium canicula Mustellus vulgaris Mustellus laevis (oviparous) (ovoviviparous) (viviparous) Egg Young Egg Young Egg Young Total weight 1.3 2.7 3.9 60.6 5.5 189.0 Organic substances 0.61 0.48 1.9 8.9 2.8 32.0 Water 0.68 2.15 1.9 49.8 2.6 152.0 mammal (哺乳類) |
|
1920-30 Spemann: completed the fate map a few years before
discovered organizer, by using hair loop, glass needle, pipette, etc. Organizer (形成体)= dorsal lip1) neural induction (神経誘導) – mesoderm, endoderm 2) organization center of embryo
neural induction Analysis for nature of induction (誘導特性解析)Technicalgrafting in vivo (A, B) → tissue (organ) culture system in vitro (C, D)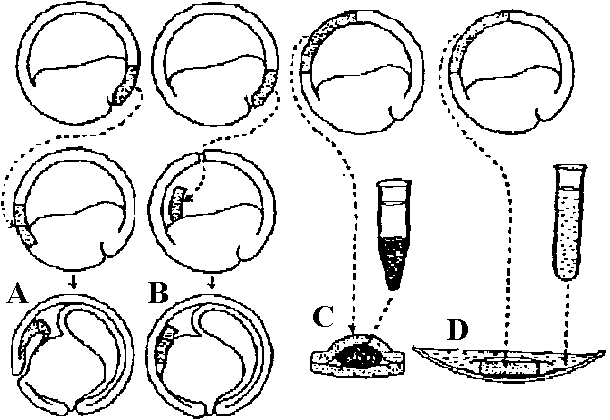 |
Figure 165. Four methods of exposing gastrula ectoderm to induction (diagrammatic). A, Transplantation of part of dorsal lip (stippled) into the ventral marginal zone, where it invaginates and forms a secondary archenteron. B, Transplantation of part of dorsal lip (stippled) into the blastocoele through a slit at the animal pole. Graft is eventually pressed against the ventral ectoderm. C, "Sandwich experiment": inductor (such as a centrifuged tissue homogenate) placed between two pieces of presumptive gastrula ventral ectoderm. D, Cultivation of a piece of presumptive ventral ectoderm of a gastrula in a watch glass filled with fluid containing inducing substance in solution. The reacting ectoderm is held between two layers of supporting material (silk) to prevent it from curling.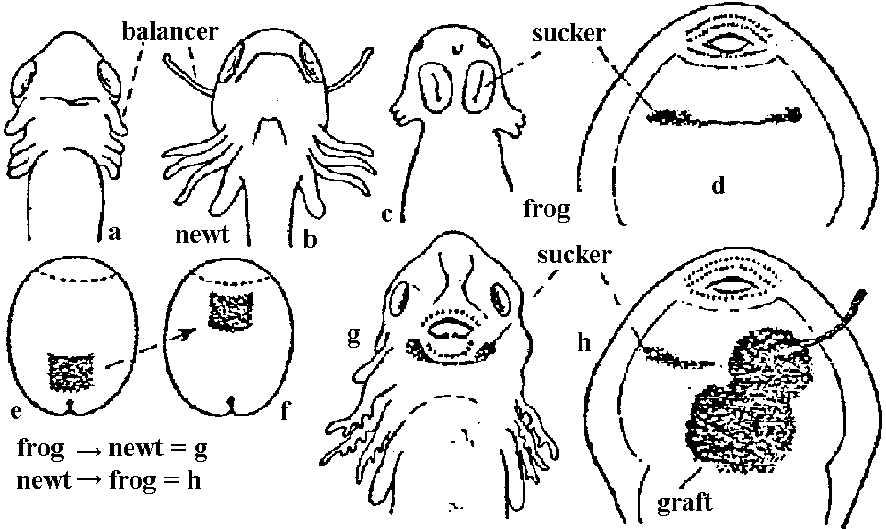 Fig. Cell-transfer experiment |
Renal system (腎臓系)developed from nephrotome, showing the relationship between ontogeny and phylogeny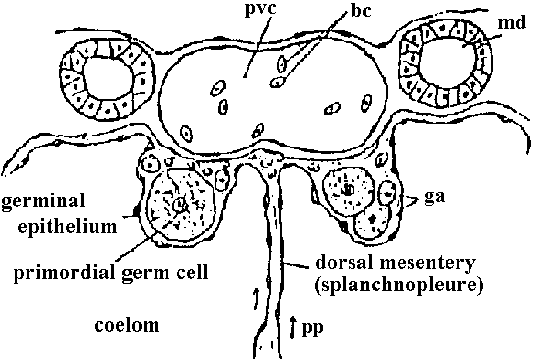 pvc: posterior vena cave, bc: blood corpuscles, md: mesonephric (Wolfflan) duct, ga: gonad anlage, pp: presumed path of primordial germ cells to gonad anlage
gonad (♂/♀) (mesodermal) ┅┅┅ different origin |
Metaphros (kidney, 腎臓)mesodermal epithelium, mesodermal mesenchyme
Pattern formation (パターン形成)1) limb field and limb area1910 Harrison: Salamander tail bud
a) destroyed 1/2 limb bud → normal limb AP-axis: neural tube closure stage / DB-axis: tail but stage Proximal-distal: forming limb (need re-consideration) 2) limb bud – polarityA-P / D-V, somatic layer mesoderm, epidermis epithelium 3) mesodermal-ectodermal interaction |
Immunity (免疫): the state or quality of being resistant to a particular infectious disease or pathogenCharacteristics of Aba) specific (特異的): antibody (Ig, or Ab = immunogloblin) vs antigen (Ag)
⇒ enzyme-substance relationship
the immune system ability to remember a previously encountered antigen and mount a faster, stronger and more specific response on re‑exposure Species (Class) Intact Light Heavy CHO(%) Designation Formula Lung fish (dipnoi) 120000 22000 38000 ND IgN L2υ2 Bullfrog (amphibian) (150000) 22000 53000 2.0 IgG? L2γ(?)2 Marine toad (amphibian) 160000 22500 53000 4.2 IgG? L2γ(?)2 Xenopus (amphibian) ND 22000 53000 ND IgG? L2γ(?)2 26700 64500 Turtle (reptile) 180000 22500 67500 IgG(Y) L2γ(Y)2 120000 22500 38000 0.9 IgN L2υ2 Sleeply lizard (reptile) 151000 22400 51000 ND IgG? L2γ(?)2 Duck (avian) 178000 22400 68000 5.0 IgG(Y) L2γ(Y)2 118000 23000 35000 0.6 IgN L2υ2 Chicken (avian) 174000 22500 67500 2.2(HEX) IgG(Y) L2γ(Y)2 ND ND ND ND IgA (L2α2) Echidna (monotreme) 150000 22500 49000 2.0(HEX) IgG L2γ2 Antigen–antibody reaction, Ag-Ab reaction (抗原抗体反応) |
Cell-mediated immunity or cellular immunity (細胞性免疫)Ab: overlays on cell surface – Ag-receptor
Humoral immune response: plasma cell → Ig circulation Genetic basis of cellular immune response
Accept Reject
1) isograft (= autograft), isogenic O
2) allograft O
3) AA × BB (P) Transplant P → F1 O
→ AB (F1) F1 → P O*
4) → AA, AB, BB F2 → F1 O
*: reject the substances that have not present in itself |
Morphogenesis (形態発生)____seed germination____↑____↓ ____↑____formation and growth of stem and root ← ____↑____↓ senescence and abscission_________↑ ____↑____↓ bud dormancy →→→→→→→→→→→ ____↑____formation of flower ____↑____↓ senescence and abscission ____↑____fruit set →→→→→→→→ seed formation ____↑____↓_________________________↓ ____↑____growth and riping of fruit______ ↓ ____←←←←←←←←←←←←←← seed dormancy |
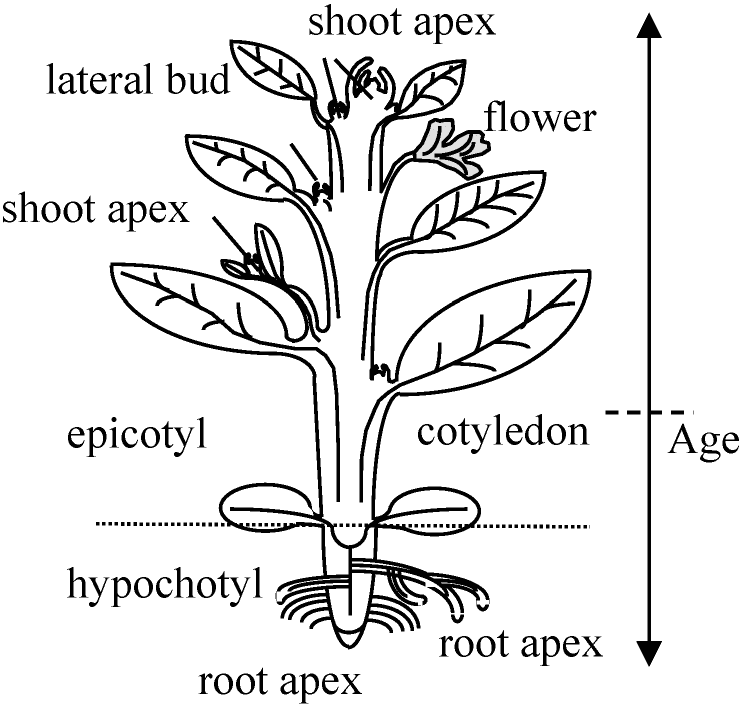
Polarity (極性)
young: shoot apical meristem (root as well as stem) Plant movement (植物の運動)Embryogenesis (胚形成) = embryo formation |
[ hormones ]
|
1959 Jacobs WP: PESIGS rules (hypothesis - not always accepted)
P (parallel variation) - correlated to physiological changes Hormonal interactionsE1 and E2: effect of horomones 1 and 2 (H1 and H2), respectivelyH1 + H2 → E1 + E2 (additive) vs E1 × E2 (synergistic) One-way action (interactions among hormones should be clarified)Ex. high concentration auxin → ethylene production↑
→ the specific enzyme(s)↑ → inactive bound auxin↑ 1. Auxin (オーキシン)IAA extraction1934 Kögl, Erxleben & Haagen-Smit (Utrecht): from human urine 1934 Kögl & Kostermans: from yeast 1935 Thimann: from Rhizopus 1946 Haagen-Smit et al.: 27-mg IAA from 100-kg immature tomato IAA crystallized - confirmed that IAA is a hormone Natural auxins = endogenous, indole acetic acid (IAA, インドール酢酸)Synthetic auxins: Ex. 2, 4-dichlorophenoxyacetic acid (2, 4-D), 2-methoxy-3, 6-dichlorobenzoic acid (dicamba) Synthesisyoung developing leavesterminal buds, growing axillary buds coleoptile tips Functionbind receptor protein in plasma membranetransport into cell activate ATPase in plasma membrane H+ ion extrusion acidify cell wall break hemicellulose-pectin bonds cellulose microfibrils slide apart cell enlarges Antiauxin (抗オーキシン): a plant substance that opposes or suppresses the natural effect of an auxin Changes in RNA metabolism induced by auxinTable. Requirement of nucleoplasimic factor for enhanced RNA synthesis by IAA (Unit = 3H-UMP incorporated (c.p.m))Incubation system (Unit) complete (336) complete - DNA (56) complete + IAA (328) nucleoplasm only (72) nucleoplasm + IAA (68) complete + nucleoplasm (412) complete + nucleoplasm + IAA (727) Table. Simulation of RNA synthesis by IAA and acceptor protein in vivo Incubation system_β, γ32P-ATP incorporated_3H-UMP incorporated ________________(pmol/mg of enzyme)____(nmol/mg of enzyme) Complete_______________________60______________6.3 Complete + acceptor protein________35______________4.2 Complete + acceptor protein + IAA__108_____________14.8 Table. Comparison of RNA polymerase activity by incubation of plasma membrane in 0.1 μM 2,4-dichlorophenoxyacetic acid, indoleacetic acid, or 3,5-dichlorophenoxyacetic acid.
[3H]-UMP incorporated (pmol/30 min/mg protein) PM, plasma membrane fraction Table. Enhancement of RNA polymerase activity by incubation of plasma membranes in 2,4-dichlorophenoxyacetic acid. Numeral = [3H]-UMP incorporated (pmol/30 min/mg protein)
RNA polymerase____________________________________ 72 PM, plasma membrane fraction Table. Effect of α-amanitin on enhancement of RNA polymerase by auxin-related factor.
+ α-amanitin* 133
RNA polymeraase 160 * α-amanitin acts as the inhibitor of mRNA synthesis. Each assay contained 2 μg of α-amanitin PLASMA CYTOPLASM NUCLEAR NUCLEUSMEMBRANE MEMBRANE Auxin ━>||━━━━> Factor ━━━>||━━> Factor + RNA polymerase ↓ Modified RNA polymerase Altered genome transcription Fig. A hypothesis in relation to the synthesis of nucleic acids operated by auxin 2. Gibberellin, GA (ジベレリン)immature seed embryo, young leaves, and roots (tissue localization) → phloem (transport)α-amylase formation induced by gibberellin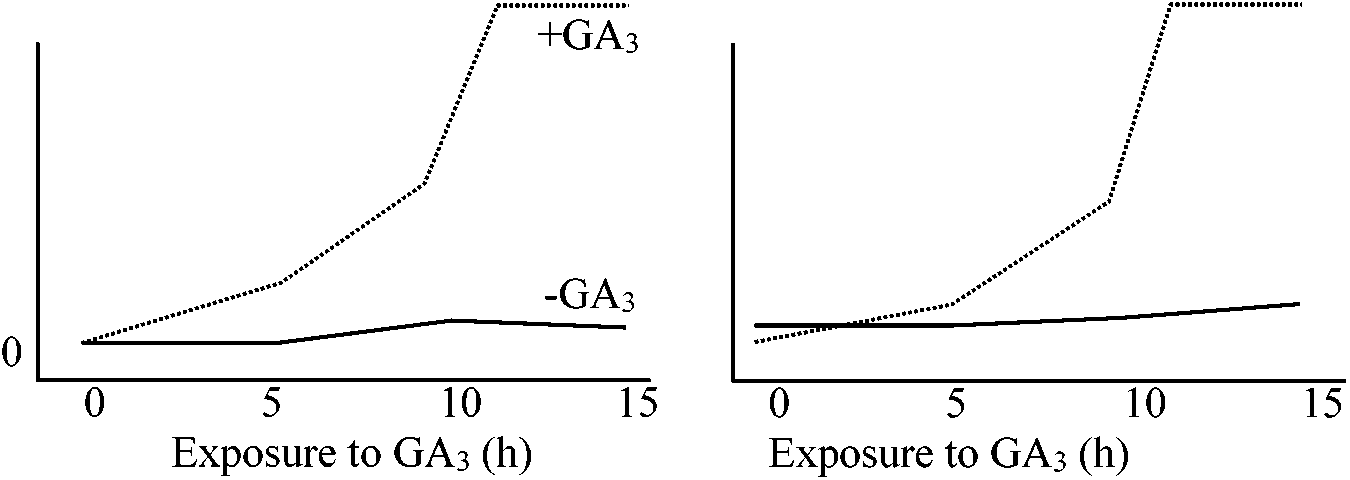 Exposure to GA3 (h) Fig. (left) α-amylase as % of total cell free protein synthesis. (right) Rate of α-amylase synthesis in vivo (U per 120 min) Biosynthesis (生合成)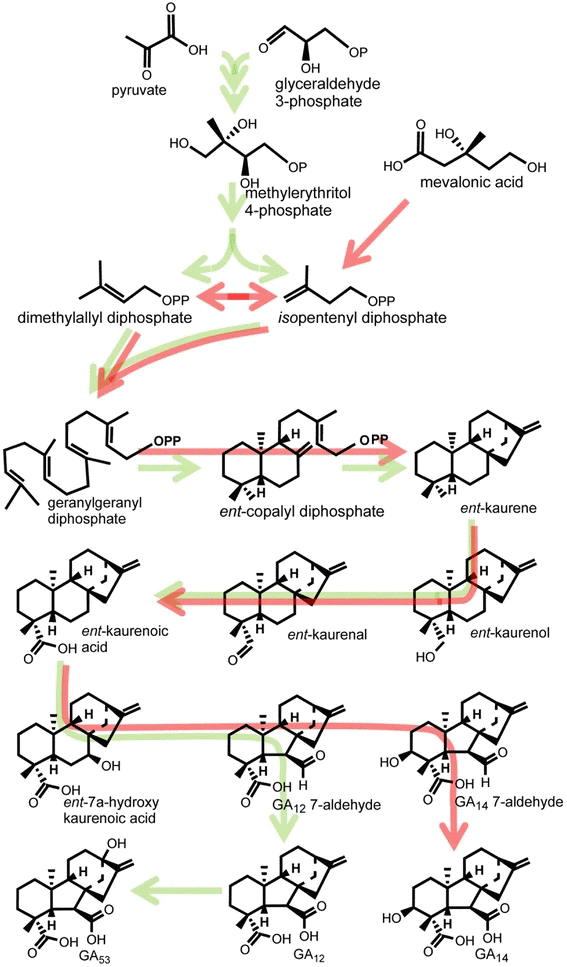 Fig. 1. Early and intermediate steps of GA biosynthesis in higher plants (green arrows) and the fungus Fusarium fujikuroi (red arrows). In plants, ent-kaurene is synthesised in plastids, predominately via the methylerythritol phosphate pathway, while in fungi, it is biosynthesised from mevalonic acid. Conversion of ent-kaurene to GA12 and GA53 (plants) and GA14 (fungi) is catalysed by membrane-associated cytochrome P450 monooxygenases. Arrows running through structures indicate multiple steps catalysed by single enzymes (Hedden & Sponsel 2015) |
3. Cytokinin (CK, サイトカイニン) (6-benzyl adenine , 6BA)Root apex (synthesis) → upward in xylem (transport)4. Abscisic acid (ABA, アブシジン酸)opposes action of gibberellin and auxinSynthesischloroplants - breakdown product of carotenoidsFunctiondormancy maintenance - high levels in dormant seeds and buds5. Ethylene (エチレン)1901-1930 discovered a gas that is physiologically active = ethylene1901 Neljubow, Dimitry: active component in illuminating gas = ethylene ethylene is the most harmful gas for plants 1910 Knight, LI: supported Neljubow1910 Cousins: orange ripnes earlier when stored with banana 1915 Harvey: some effects of ethylene on the metabolism of plants 1930 Chase: unripe, green pears softens fast by mature pear products 1932 Elmer: abnormal germinaton of potato indcued by a volatile element in matured apple 1933 Kidd & West: a gas promotes ripning of fruits1934 Gane, Richard: biosynthesis of ethylene by plants 1935 Crocker, William; ethylene = plant hormone 1947 Miller: published "The story of ethylene" Burg, Stanley P
1959 research on ethylene biosynthesis trade name = ethrel → applying research on gardening 1984 Knee: climacteric peak - respiration↑ during maturation
Etylene production (nl/g/hr) on climacteric plants slightly different among the varieties and/or cultivars
Etylene production (nl/g/hr) on non-climacteric plants ConditionsTemperature: affects C2H4 synthesis largelyEx. apple: Q10 = 2.7 (0-10°C). 2.8 (10-25°C), 1.74 (10-30°C) ⇒ break point on Arrhenius plot → phase transition (sometimes 11.4°C) Air: O2↑ → promoting maturation. CO2↑ → supressionO2 is required for ethylene production Contact stimulus (thigmomorphogenesis): trigger of ethylene productionEx. wheat treading (麦踏), classically known 1971 Matsukawa (松川) et al. Lilium longiflorum
grew short when the shoots were swept by hand or brush most plants supressed growth ⇒ thigmomorphogenesis Disease contraction1950 Williamson CE: diseased plants produced much etylene 1953 Dimond AE: Fusarium oxysporum f. sp. lycopersici (トマト萎凋病) → epinasty (上偏成長), induced by ethylene Table 1. Plant responses to ethylene (Saltveit 1999)
Inhibition responses ethylene synthesis (positive feedback) fruit ripening pigment synthesis cholorophyll destruction seed germination adventitious root formation respiration abscission senescence Biosynthesis1954 Fergus: Penicillium digitatummannose, mannitol, citric acid → ethylene 1957 Phan-Chon-Ton M: P. digitatum, glycerol, alanine → ethylene↑1964 Lieberman M, Mapson LW: genesis and biogenesis of ethylene
|← hemicellulose
1: S-adenosyl methionine
C1 ⇒ carbon dioxide (CO2)
methionine NH3 1979 Adams & Yang: apple fruit + ACC → ethylene these two experiments cofirmed: ACC = precursor of ethylene Ethylene produced not only by plants but also animals, bacteria and fungiMetabolism1957 Buhler et al.: mature avocado + green pear + radioactive ethylene
ethylene (14C2H4) abosorved by plants → confirmed the uptake 1964 Jansen et al.: avocado + radioactive etylene → uptake by benzene and toluene 1969 Shimokawa (下川敬之) et al.: juvenile of purple morning gloryradioactive etylene → not moved for long distance Beyer et al.: ethylene metabolism examined by pea juveniles
/O\ Ethylene in soil (土壌中エチレン)1974 Lynch: Mucor hieralis, soil bacterium - producing ethylene1976 Smith A: ethylene oxygen cycle (酸素-エチレンサイクル)
[plant exudes sugars] ⬅ [nutrients rebind to clay
adjust energy turnover and nutrient use efficiency f) Brassinosteroid (BRs/BR, ブラシノステロイド)1970s: Mitchell et al.: organic extracts of Brassica napus pollenpromoted stem elongation and cell division Functioncell expansion and elongation (working with auxin)cell division and cell wall regeneration (unclear and controversial) promoting vascular differentiation pollen elongation for pollen tube formation acceleration of senescence (in vitro) protecting from chilling and drought stress 7. Florigen (フロリゲン)Hormonal control at hormone levelAuxin-induced ethylene production - regulation of ethylene biosynthesis -
RA + RB = RAB ← additive response (R: response, AB: hormones) |
Somatic division or somatic cell division (体細胞分裂)1953 Pelc & Howard
G1 (gap1) → S (synthesis) → G2 (gap2) → M (mitosis) prophase → metaphase → anaphase → telophase Meiotic division |
|
Def. the scientific study of biological rhythms — the time-based patterns in physiological and behavioral processes of living organisms
Determinants: light, temperature, tides, etc.
Table. The activity type
term
active time
remarks Photoperiodicity or photoperiodism 光周性1. Phytochrome (ファイトクローム)1935 Flint & McAlister: seeds from a cultivar of lettuce germination rate
a few percent under dark exposed to radiation of which wavelengths ≥ 700 nm (far-red, FR) → inhibited seed germination (Institute in Beltsville, Maryland) 1959 Butler WL et al.: isolated and identified from seedlings
Pr ↔ Pfr |
1952 Bothwick et al.: seeds of lettuce (cv. Grand Rapids
examined the effects of R/FR on seed germination (this cultivar does not germinate the seeds under the darkness)
R: exposed to red at 1 W/m2 for 1 min
light treatment R +FR +R +FR +R +FR +R R: induced seed germination ⇔ FR: inhibited seed germination 1960 proposed the term phytochromeRef. Mycochrome Table. The effects of blue (B) and near-ultraviolet (NUV) on the spore formation of Helminthosporium oryzae (ごま葉枯病菌, Syn. Cochliobolus miyabeanus) (Honda et al 1968)
Dark(control) NUV : B +NUV +B +NUV +B +NUV ⇒ presence of mycochrome |
|
1968 Harris H
Ⓧ + ◎ + UV irradiation →
the most of energy during the emergence process is from protei. carbonhyborates are used least |
1972 Firtel RA
3H-DNA → thermal denaturation for single strand → sharing force, ca 400 nucleotides → remnant, return to double strand (= repeated sequence) → hydroxyappite column → probe DNA-RNA hybrid
gr aggr pseudo culm all gr + culm gr = growth. aggr = aggregation. culm = culmination. pseudo = pseudo-plasmodium. all = all stage
Growth + (Growth + Culmination) = 65.8: common genes present Key enzyme (キーエンザイム)UDP-galactose (polysaccharide) transferase: spore-specific substancesenzymes related to carbohydrate synthesis (cf. PSV: prespore vacuole) - absent in the stalk Ex. Blastocladiella emersonii (水生菌), Acetabularia mediterraneanuclear-cytoplasm interaction after the nuclear transfer Higher plants (高等植物)seed: analogy of animal embryoEx. α-amylase / β-amylase de novo: gene = active → m-RNA → protein (α-amylase) |
Double fertilization and division pattern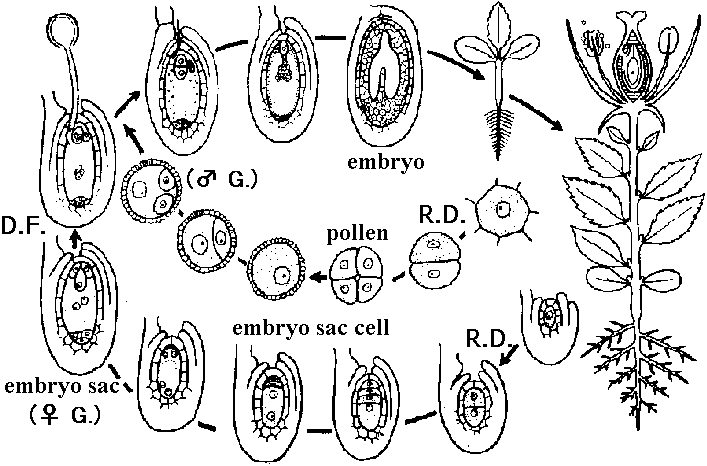 Fig. Life cycle of Angiospermae (Engler’s Syllabus 1964) 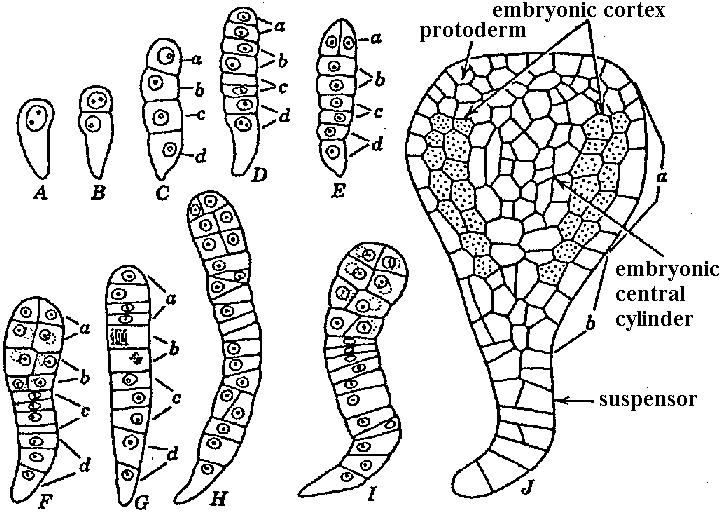 Fig. Embryo development in Daucus carota. Longitudinal sections. The lower end of embryo in each drawing is the end directed toward micropyle. A-C, stages in development of linear four-celled embryo. D, E, two common variations in eight-celled embryos: difference in division of cell a of the four-celled embryo. F-I, older embryos varying in cell arrangement. J, embryo differentiated into main body and suspensor. Initial organization of tissue regions is present in J. Relation of parts of certain embryos to cells of the four-celled embryo (C) is indicated by the letters a-d. (Borthwick 1931). |
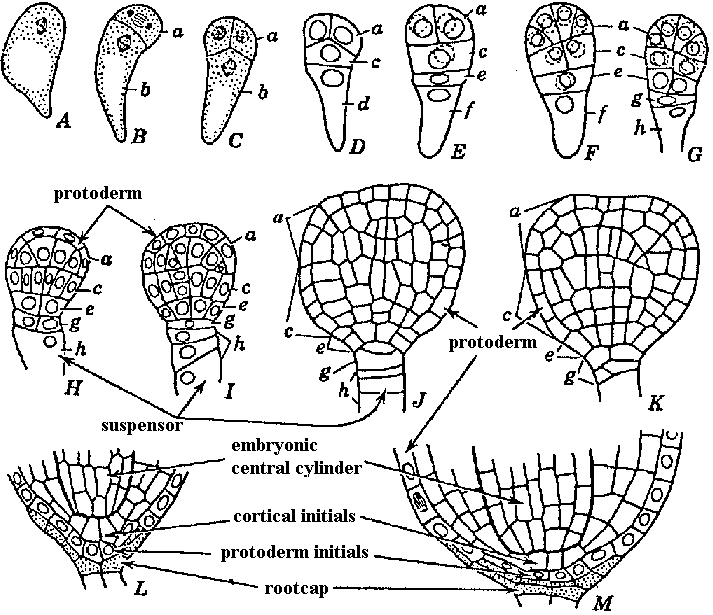 Fig. Embryo development in Lactuca sativa (lettuce). Longitudinal sections. Lower end of embryo in each drawing is the end directed toward micropyle. A, zygote in division. B-G, embryos in successive stages of development, showing establishment of several horizontal tiers of cells. In G, cell h later gives rise to all of the suspensor cells, and the tiers above h develop into the main body of embryo. H-M, further development stages. |
embryo: gibberellin synthesis after immersion → active α-amylase synthesis → auxin → differentiationα-amylase (α-アミラーゼ)EC 3.2.1.1hydrolyses alpha bonds of large, α-linked polysaccharides, e.g., starch and glycogen, yielding short chains, dextrins and maltose |
|
1978 Kreibich G, Ulrich BL & Sabatini DD: Ribophorin in pea epicotyl Ribophorin in pea epicotyl
Free
Membra
ne
Total amount is constant - (possibility) free-bound induced by hormone
Heme = globrin, Hemine |
Elongation factor (伸長因子)1972 Tome et al.: Pea ribosomes: poly(U)-14C-phenyl alanine
2 days 4 6 |
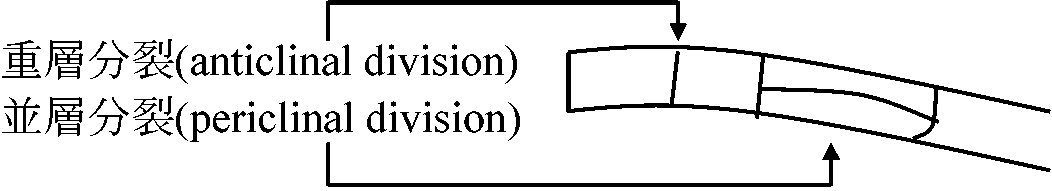 (☛ shoot apex) (☛ shoot apex)
anticlinal division (垂層分裂): cell division occurring in a plane perpendicular to the surface of the tissue or organ
Chimera or chimaera (キメラ)Originating from ancient Greek word Chimaira (= she-goat or a fantastical creature)the Chimera was a monstrous hybrid creature with the body of a lion, the head of a goat rising from its back and a tail ending in a the head of serpent. It breathed fire and symbolized a terrifying and unnatural combination. Def. (botany): a single organism that contains genetically distinct tissues originating from different zygotes |
Cellular chimera (細胞キメラ)= chimera (s.s.)a state where different types of cells coexist within the same individual Relationships between stem and root (茎と根の関係)1950 Skoog: Tobacco pith
IAA 0.002 ppm → root
_____________Callus___Roots___Leaves
1) embryoid (胚様体) tobacco pith cells → culture single cell → can form both bud and root 1965 Chen & Gaglston: Pelargonium pith collecting from pith → callus
medium: auxin, kinetin + agar → to liquid: differentiate root, stem and leaf 1971 Nagata & Takebe (長田・建部): Tobacco mesophyll protoplast → mature plant/embryoid → callus mass |
Defoliation (落葉)C2H4: promoting cellulase in the abscission layer (or zone)
Lamina senescence____Abscission layer(zone)
cell differentiation ┌ plant hormones |
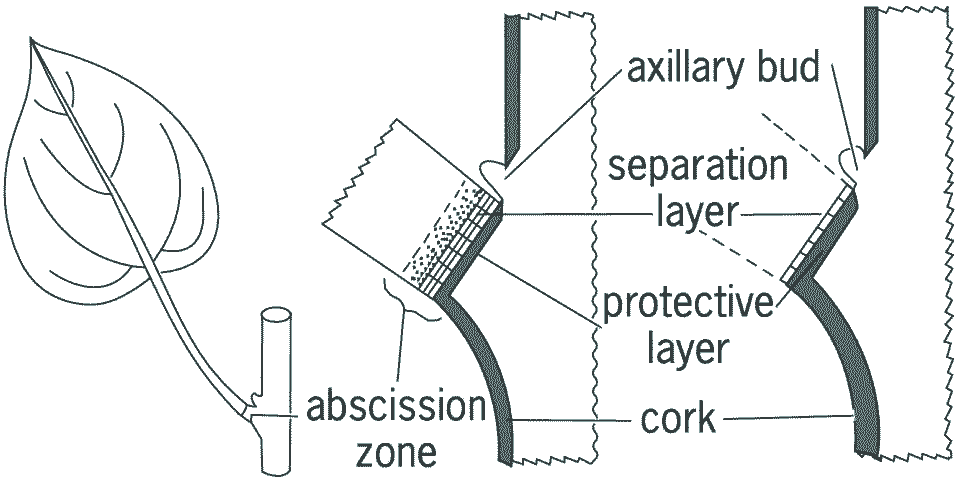 (a) (b) (c) Diagrams of the abscission zone of a leaf. (a) A leaf with the abscission zone indicated at the base of petiole. (b) The abscission zone layers shortly before abscission and (c) the layers after abscission. Flower shedding (落花)Fruit shedding (落果) |
ABC model Fig. ABC model of flower development guided by three groups of homeotic genes. |